
April 3, 2018
A Design Input Specification (DIS) plays a critical role in any product design process.
Investors and the FDA do for sure.
Investors want as little technical uncertainty as possible, and the FDA wants proof that your product will do the job as intended. On a serious note, the FDA will review EVERYTHING as it is a mandatory regulatory condition in our world for medical device development.
Detailed design input specifications make your design team's job easier -- which makes YOUR job easier.
Your DIS is a living document that will shift and move as you journey along the path to commercialization.
Far from a rigid, frozen document, the DIS is a tool that needs to be used by teams to foster innovation and should lead to greater confidence in the final deliverables.
Here are six approaches you can use to generate inputs. Following afterward is a real example of how these approaches look in practice.
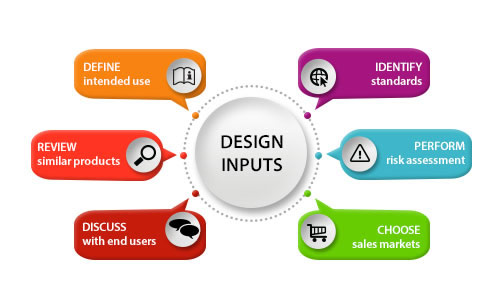
This activity will vary greatly depend on whether this is an incremental improvement to an existing product, or a brand new direction.
As a team, consider where it will be used, how it will be used, and who is expected to use it.
Consider if it needs to be sterile, single use, needs to conform to a specific procedure, etc.
If you are looking to compete directly against a known competitor, this can be fairly straightforward. Often, you can find published instructions for use; which will contain basic specifications.
Leverage the work of others to gather inputs on mandatory items. Learn what trade-offs other teams have made.
Depending on your goals, you can learn what your team must achieve to be an equivalent medical device or what they must strive for if you are introducing the next best device.
If you are working on something truly innovative, you may still get some good ideas from the design elements of existing complementary products that are used in the same environment.
If possible, observe the procedure or interactions of your proposed device's environment. This can be a challenging and expensive task, but arguably the most influential.
These interactions can range from informal to formal.
You are looking to reduce uncertainty and build confidence to foster the creativity required in the ideation phase of design, which will follow.
Make a list of the needs in clear and simple language.
As a team, review the general, collateral, and specific standards that apply to your product, and extract the requirements needed to achieve conformance.
I recommend using the FDA's Manufacturer and User Facility Device Experience database (MAUDE) to review common failures associated with the product class within which you are designing.
This can also be useful for detecting trends in similar devices. These trends can become inputs for your design team to consider.
Once you have selected your markets, you can start to classify your device in each of them. The USA, Europe, and Canada share similarities, but each will have some unique requirements.
Knowing your market locations and their regulatory standards ahead of time will affect the level of detail required for your DIS.
Defining design input specifications doesn't have to be a drag. Come up with creative ways to get the team brainstorming and gain maximum benefit from this important design tool.
One big tip I cannot stress enough is: Make your DIS usable! The level of detail should match the level of complexity and risk. You'll thank yourself later.
In practice, your DIS generation may look like the following excerpt from the DIS of a stent for the superficial femoral artery.
Based on defining use, we generated these possible design inputs:
Reviewing currently marketed products can allow you to quickly determine the range of stent sizes available for this artery.
Based on reviews, it was determined that common stent lengths range from 20mm to 200mm. Therefore, depending on our goals, a design input will specify that the stent length must be as good as or better than competitors.
While observing a stent placement procedure it was determined that backlash during stent deployment can negatively impact correct placement.
An input for establishing limits for unwanted movement during deployment will increase user satisfaction and improve patient outcomes.
A review of the ISO standard ISO 25539-2:2012 Cardiovascular implants - Endovascular devices - Part 2: Vascular stents would be required. We use this information to generate more inputs.
A search of the FDA's MAUDE database reveals that for 'Product Code: NIP' and 'Event Type: Malfunction' reveals that a fairly common occurrence within the last 5 years is incomplete stent delivery or partial stent deployment.
Based on this information, an input should be developed to address primary deployment system failure, like having a secondary deployment system as a backup.
If your selected market is the USA only, you would be required to conform to the FDA's 21 CFR Part 820. The device class for a stent, Superficial Femoral Artery is class 3, which becomes one of our design inputs.
As a medical device consultant, I offer up a wealth of knowledge and guidance, especially in Design Input Specification development.
I invite you to connect with me for a discussion. I am also available to hear any concerns you may have or to review your DIS if you want an outside perspective.

© 2019 Koven Technology Canada Inc.